SKU:
110360
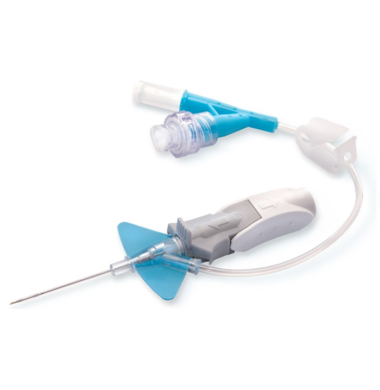
✓ Catheter’s stabilization platform minimizes movement that can lead to catheter complications, restarts and associated costs
- Built-in stabilization platform and pre-attached extension tubing
- Reduces dislodgement by 84%
- Meets the INS Standards and CDC Guidelines for catheters stabilization
✓ Proprietary BD Vialon™ Biomaterial enables longer dwell times, supporting the current infusion Nursing Standards of Practice for clinically indicated site rotation.
- In a 2014 clinical study, it was demonstrated that BD Nexiva™ catheters had a low rate of phlebitis and a median dwell time of 144 hours for catheters in place ≥ 24 hours
- In a 2014 clinical study, the longer dwell times of closed systems led to a cost reduction of approximately $1M/year/1,000 beds compared to a system
✓ Designed to minimize treatment delays and complications.
- Power injection compatible, minimizing the need to exchange catheter or tubing
- Single and dual ports and 18 - 22 gauge bore tubing are indicated for use in CT power injection
- Avoid leaking at the catheter/extension set connection due to the integrated system
✓ Integrated systems need fewer add-on devices, minimizing the number of manipulations which may lead to touch contamination and accidental disconnections
✓ BD Instaflash™ Needle Technology quick blood visualization may help improve insertion success, and therefore reduce insertion attempts
✓ BD Vialon™ Biomaterial has been clinically demonstrated to dwell up to 144 hours.
PRINT THIS IMAGE
 Philippines Toll Free: 1-800-1-888-555
Philippines Toll Free: 1-800-1-888-555